Raw problem statement:
find moles of acid reacted and moles of water produced HC2H3O2+NaOH>H2O+NaC2H3O2
Additional information given on clarification:
Solution:
First of all, let's decode the formula.
The first ingredient, HC2H3O2, is Acetic Acid, or simply vinegar, it molecule look like this.
The second ingredient, NaOH, is Sodium Hydroxide, commonly known as caustic soda.
Sodium ion and hydroxide ion are bind together through and ionic bond.
Caustic soda is a base, so this is acid + base neutralization reaction.
The reaction formula itself is balanced, therefore to compute the number of moles, we use the input output relationship.
The formula is already balanced, so every unit of acid consumed will produced an unit of water, so the answer is equal.
Finally, we have 50ml of 2.0 M solution, we multiply them (in SI units) to get $ 0.05 \times 2.0 $ = 0.1 mole.
We have 0.1 mole of acid and 0.1 mole of base, so all of them will be consumed and produce 0.1 mole of water and salt.
That's it!
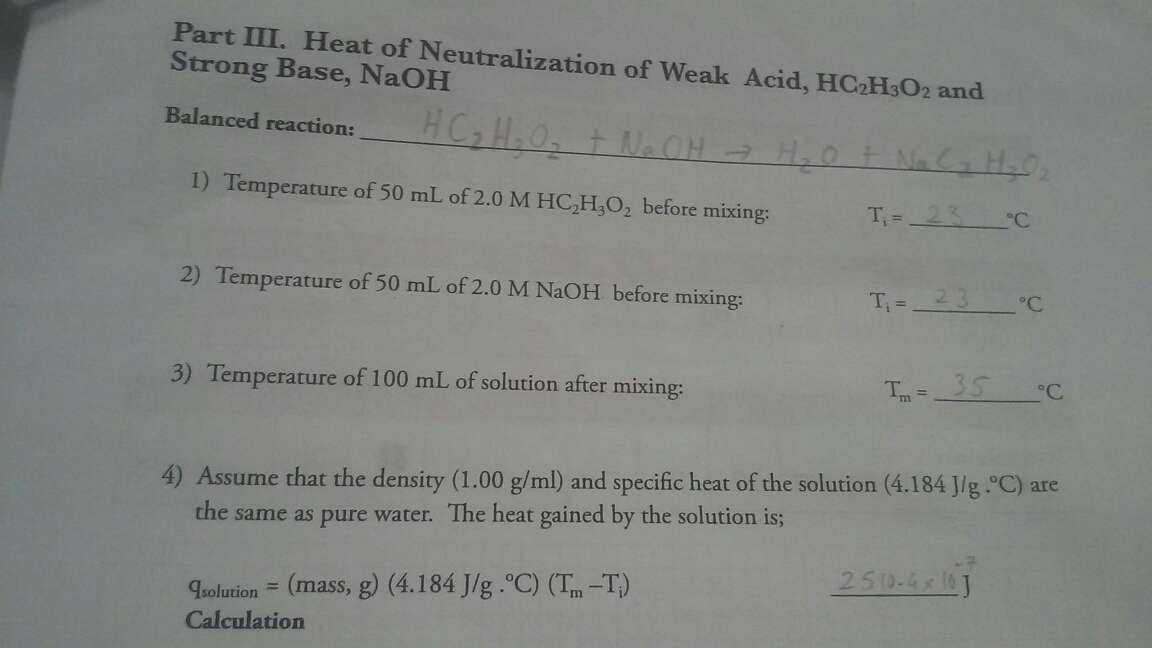
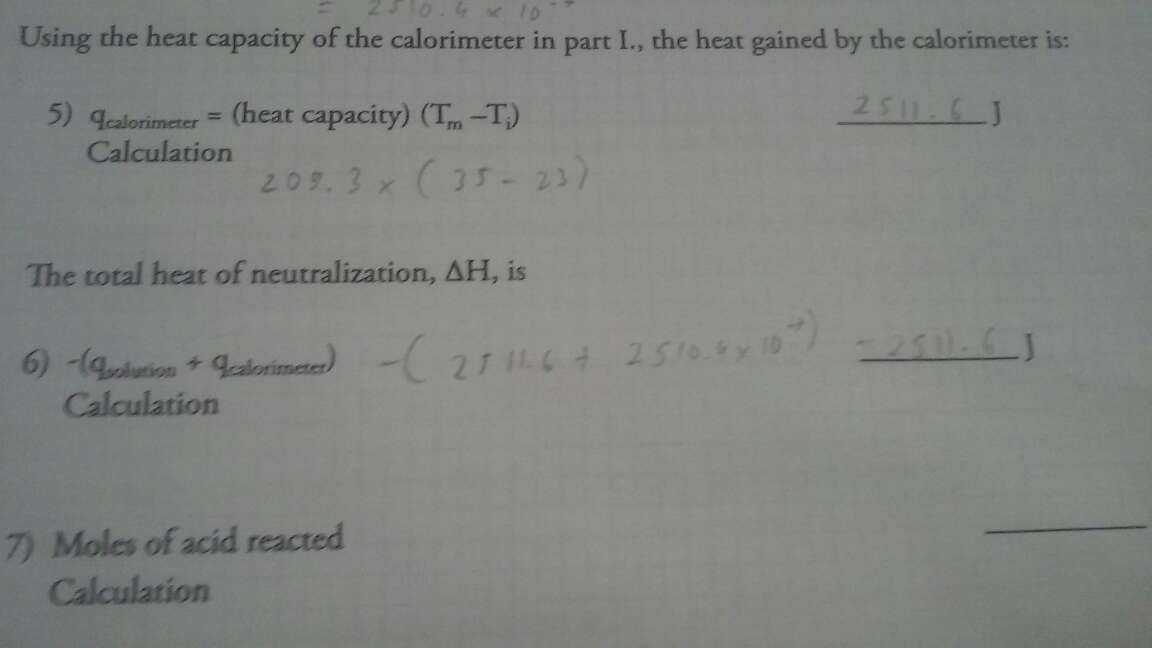
No comments:
Post a Comment