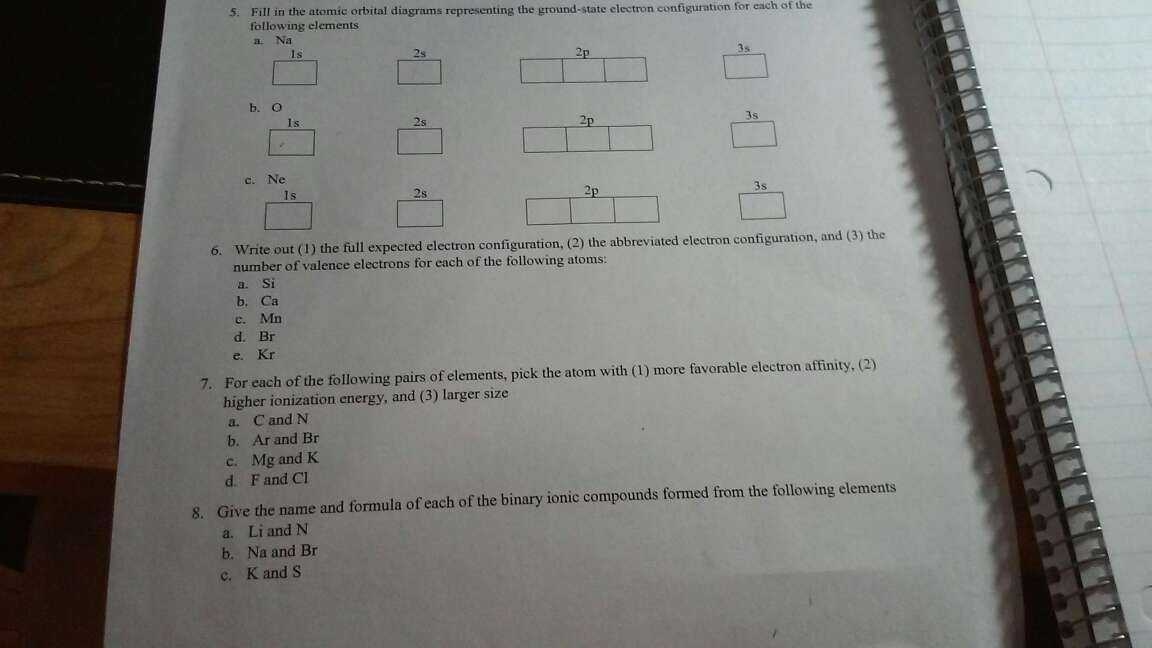
Solution:
Question 6
Full
|
Abbreviated
|
Valence
|
|
Silicon
|
$1s^2 2s^2 2p^6 $
|
[Ne] $ 3s^2 3p^2 $
|
4
|
Calcium
|
$ 1s^2 2s^2 2p^6 3s^2 3p^2 4s^2 $
|
[Ar] $ 4s^2 $
|
2
|
Manganese
|
$ 1s^2 2s^2 2p^6 3s^2 3p^2 4s^2 3d^5 $
|
[Ar] $4s^2 3d^5 $
|
7
|
Bromine
|
$ 1s^2 2s^2 2p^6 3s^2 3p^2 4s^2 3d^{10} 4p^5 $
|
[Ar] $ 4s^2 3d^{10} 4p^5 $
|
7
|
Krypton
|
$ 1s^2 2s^2 2p^6 3s^2 3p^2 4s^2 3d^{10} 4p^{6} $
|
[Kr]
|
8
|
Question 7
|
|
+ electron affinity
|
+ Ionization energy
|
+ size
|
|
Carbon and Nitrogen
|
Nitrogen
|
Nitrogen
|
Carbon
|
|
Argon and Bromine
|
Argon
|
Argon
|
Bromine
|
|
Magnesium and Potassium
|
Magnesium
|
Magnesium
|
Potassium
|
|
Fluorine and Chlorine
|
Florine
|
Florine
|
Chlorine
|
$ Li_3N $
$ NaBr $
$ K_2S $
No comments:
Post a Comment