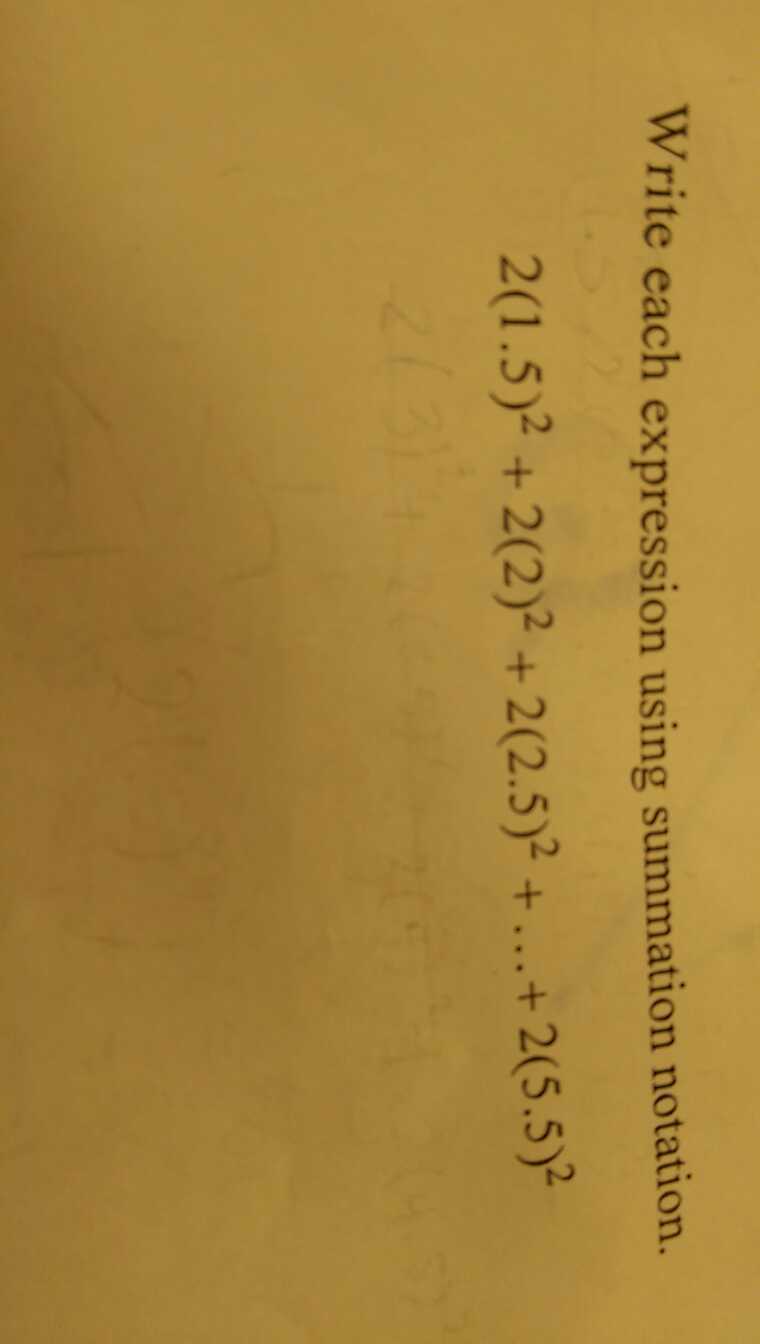Problem:
 Solution:
Solution:
Let's determine what are the chemicals first.
$ CH_3CO_2H $ is acetic acid, it is a weak acid in aqueous solution.
$ NaCH_3CO_2 $ is sodium acetate, it is a salt.
$ HCl $ is hydrochloric acid, of course.
First of all, we have 100 mL = 0.1 L of the buffer solution, so we have
$ 0.2 \times 0.1 = 0.02 $ mol of $ CH_3CO_2H $ and
$ 0.34 \times 0.1 = 0.034 $ mol of $ NaCH_3CO_2 $.
The salt is an ionic compound, so it dissolves in water and become $ Na^+ $ and $ CH_3CO^-_2 $
The fact that acetic acid is a weak acid means we need to compute how well it dissociate in water using the constant, in particular, we have these two equations
$ pK_a = -\log K_a $
$ K_a = \frac{[A^-][H^+]}{[HA]} $
With the first formula, we can solve for $ K_a = 10^{-4.74} $.
Now we have the reversible reaction:
$ CH_3COOH \to CH_3COO^- + H^+ $, let say, out of 0.02 mol of $ CH_3COOH $, $ p $ mol of them got dissociated, so we get the total number of moles of elements as:
$ \begin{eqnarray*} [CH_3COOH] &=& 0.02 - p \\ [CH_3COO^-] &=& p + 0.034 \\ [H^+] &=& p \\ [Na^+] &=& 0.034 \end{eqnarray*} $
Plug these values into the $ K_a $ formula, we can solve for $ p $ as follow:
$ 10^{-4.74} = \frac{(p + 0.034)(p)}{0.02-p} $
The resulting quadratic formula has two roots, rejecting the obviously wrong negative solution, we have solved $ p = 1.0695 \times 10^{-5} $.
So the pH value is $ -\log p = 4.97 $.
Now we add hydrochloric acid. We added 8ml = 0.008L of 1M $ HCl $, so we have added $ 0.008 \times 1 = 0.008 $ mol of $ HCl $.
Hydrochloric acid is a strong acid and we assume it completely dissociate (not completely true, but a good approximation), so the number of moles changes as follow:
$ \begin{eqnarray*}
[CH_3COOH] &=& 0.02 - p \\
[CH_3COO^-] &=& p + 0.034 \\
[H^+] &=& p + 0.008 \\
[Na^+] &=& 0.034 \\
[Cl^-] &=& 0.008 \\
\end{eqnarray*} $
Now we solve the $ pK_a $ formula again and get $ 10^{-4.74} = \frac{(p + 0.034)(p + 0.008)}{0.02-p} $, but wait, the formula only generate negative $ p $. Now, what does it mean? It means instead of dissociating, the acetate ions take the proton and form the acid instead! In this case, we have $ p = -0.00798 $ and therefore the proton concentration is $ -0.00798 + 0.008 = 1.95684 \times 10^{-5} $, so the pH is $ -\log (p + 0.008) = 4.71 $.
As expected, it becomes more acidic!